|
| Thread: Molecule on microscope... | |
|
Seraphim

 
    
Supreme Hero
Knowledge Reaper
|
 posted September 16, 2012 02:45 PM
posted September 16, 2012 02:45 PM |
|
Edited by Seraphim at 20:38, 16 Sep 2012.
|
Molecule on microscope...
Quote:
A team of IBM scientists--known for capturing the first close-up image of a single molecule in 2009--now have revealed incredibly detailed microscopic images that show the individual chemical bonds between atoms.
Link
Quote:
"In the case of pentacene, we saw the bonds but we couldn't really differentiate them or see different properties of different bonds," Gross told BBC News. "Now we can really prove that... we can see different physical properties of different bonds, and that's really exciting."
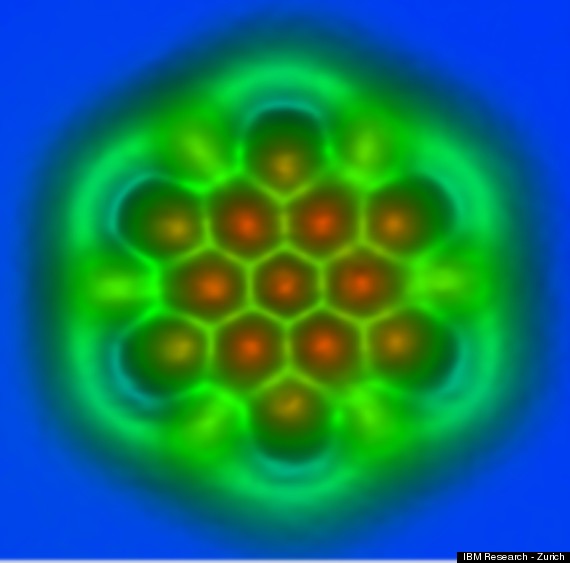
Nanographene as a replacement for Silicon microchips is already being experimented.
With graphene's lower noise, we might have better and faster PC's in the future with even more MHZ per core.
Quote:
These images not only illustrate the structure of individual nanographene molecules but also how atoms are bound together.
In case you dont know what graphene is, it is an allotrope of Carbon.
Quote:
Graphene appears to be one of the strongest materials ever tested. Measurements have shown that graphene has a breaking strength 200 times greater than steel.
Graphene has the ideal properties to be an excellent component of integrated circuits. Graphene has a high carrier mobility, as well as low noise, allowing it to be used as the channel in a field-effect transistor.
It seems that with tomorrow's technology, we will be having mainframes as pocket computers.
Edit:
I would not expect nobody replying to this thread. Its awesome to see HD picture of a molecule and its structure through a microscope.
|
|
Zenofex

   
     
Responsible
Legendary Hero
Kreegan-atheist
|
 posted September 17, 2012 06:42 PM
posted September 17, 2012 06:42 PM |
|
|
So these things inside the cells are atoms, right?
Anyway, a more interesting question would be "is this how a molecule actually looks like"? What's the role of these colours and are they added later just to illustrate something? And how many dimensions does this thing have*?
*The last question's related to certain... speculations backed with arguments (due to lack of better expression) that I read a while ago that the smallest particles aren't 3D.
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 17, 2012 08:02 PM
posted September 17, 2012 08:02 PM |
|
Edited by Corribus at 20:03, 17 Sep 2012.
|
Zenofex
Quote:
So these things inside the cells are atoms, right?
No, the atoms are at the vertices of the green lines (forming hexagonal patterns). The colors are likely false, artifically colored for better contrast. Probably they represent electronic density or some such - so that chemical bonds (bright green) can be visualized. Hard to know without a legend.
Quote:
And how many dimensions does this thing have*?
Graphene is a flat, two dimensional "molecule". Essentially it's a 2D version of diamond, although with very different electronic properties because of the bonding structure.
____________
I'm sick of following my dreams. I'm just going to ask them where they're goin', and hook up with them later. -Mitch Hedberg
|
|
Seraphim

 
    
Supreme Hero
Knowledge Reaper
|
 posted September 17, 2012 09:08 PM
posted September 17, 2012 09:08 PM |
|
Edited by Seraphim at 21:12, 17 Sep 2012.
|
Quote:
So these things inside the cells are atoms, right?
Anyway, a more interesting question would be "is this how a molecule actually looks like"? What's the role of these colours and are they added later just to illustrate something? And how many dimensions does this thing have*?
To better illustrate what actually you see on those pcitures:
Look at this:
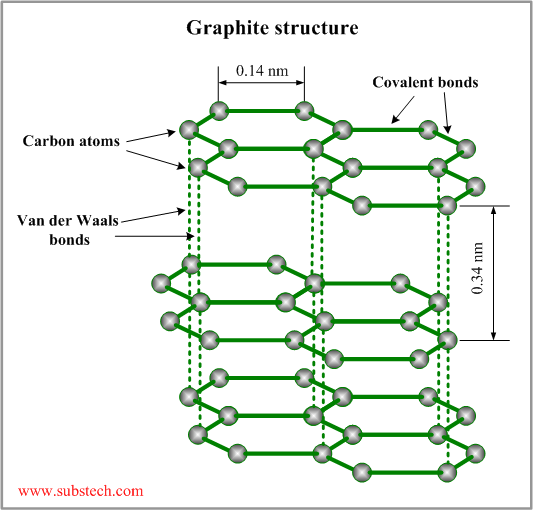
http://upload.wikimedia.org/wikipedia/commons/thumb/9/9e/Graphen.jpg/750px-Graphen.jpg
Each "Link" or "Ball" is an atom.
Here, even a better picture:
The colors, as pointed out by friendly corribus, are most likely false color.
Quote:
*The last question's related to certain... speculations backed with arguments (due to lack of better expression) that I read a while ago that the smallest particles aren't 3D.
I doubt so. Every particle is depicted in 3D as is all observable matter.
|
|
blizzardboy

    
      
Honorable
Undefeatable Hero
Nerf Herder
|
 posted September 18, 2012 02:02 AM
posted September 18, 2012 02:02 AM |
|
|
I heard rumors that nanographene eventually has to be successfully implemented into computer hardware, otherwise they'll hit a rock wall in the R&D of the industry. Silicon is reaching its limits on what it can handle.
____________
"Folks, I don't trust children. They're here to replace us."
|
|
Seraphim

 
    
Supreme Hero
Knowledge Reaper
|
 posted September 18, 2012 02:48 AM
posted September 18, 2012 02:48 AM |
|
|
Quote:
I heard rumors that nanographene eventually has to be successfully implemented into computer hardware, otherwise they'll hit a rock wall in the R&D of the industry. Silicon is reaching its limits on what it can handle.
The will hit a rock anyway. Around 2020. Once they molecular/atomic integreation is achieved, moore's law ends(18 months==Double-ing of the speed of computers).
It is physically impossible to create transistors smaller or the size of an atom. Reason: Heat issues.
According to this: http://www.sciencedaily.com/releases/2012/02/120219191244.htm
Quote:
The single-atom transistor does have one serious limitation: It must be kept very cold, at least as cold as liquid nitrogen, or minus 391 degrees Fahrenheit (minus 196 Celsius).
Of course graphene may become the new main component, but atomic "transistors" are the end of moore's law no matter what they discover.
Also,graphene is hard to produce which means that it cant replace anything silicon based for the next 20 years.
Bad news people...
|
|
blizzardboy

    
      
Honorable
Undefeatable Hero
Nerf Herder
|
 posted September 18, 2012 03:18 AM
posted September 18, 2012 03:18 AM |
|
|
Yeah, it means I might not have to buy a new computer every 2-3 years to keep up with the damn system requirements of stuff 
____________
"Folks, I don't trust children. They're here to replace us."
|
|
dimis

   
    
Responsible
Supreme Hero
Digitally signed by FoG
|
 posted September 18, 2012 03:56 AM
posted September 18, 2012 03:56 AM |
|
|
Moore's law is over already. Almost all of the speedup currently achieved is due to parallelism on software, rather than on advances on hardware. Not everything can be parallelized, so game over.
____________
The empty set
|
|
blizzardboy

    
      
Honorable
Undefeatable Hero
Nerf Herder
|
 posted September 18, 2012 04:27 AM
posted September 18, 2012 04:27 AM |
|
|
Why is it visualized in a delicious honeycomb pattern? Is that diagrammatic or is that how the atoms are arranged?
____________
"Folks, I don't trust children. They're here to replace us."
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 18, 2012 04:33 AM
posted September 18, 2012 04:33 AM |
|
|
It's how the atoms are arranged. To explain why they adopt a hexagonal pattern would require some foray into quantum chemistry and I doubt anyone here wants to read through that. Most simply put, hexagon is one very stable structure for carbon atoms can adopt (another is tetrahedra, as in diamond). The simplest form of this structure is found in benzene. We refer to the property of carbon stabilization in these kinds of structures as "aromaticity" (although not all aromatic carbon compounds adopt hexagons).
____________
I'm sick of following my dreams. I'm just going to ask them where they're goin', and hook up with them later. -Mitch Hedberg
|
|
Fauch

   
      
Responsible
Undefeatable Hero
|
 posted September 18, 2012 05:34 PM
posted September 18, 2012 05:34 PM |
|
Edited by Fauch at 17:34, 18 Sep 2012.
|
we can conclude that former heroes games had it right! 
|
|
Seraphim

 
    
Supreme Hero
Knowledge Reaper
|
 posted September 18, 2012 06:48 PM
posted September 18, 2012 06:48 PM |
|
Edited by Seraphim at 18:49, 18 Sep 2012.
|
Quote:
we can conclude that former heroes games had it right! 
Even saturn agrees on that...
Link
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 26, 2012 04:37 PM
posted September 26, 2012 04:37 PM |
|
|
|
Here is a blurb article at C&EN about this study. I don't think you need to be a subscriber to access it.
|
|
|
|





